Oh, I do love it when a theoretician comes in from the dugout and turns the game around. Billy Beane did that for the Oakland baseball team (see Moneyball, the book or the movie). Now a team led by James Stevenson in Dr Paulette Clancy’s lab at Cornell has done it for the scientific game of “What’s Life?”
Yeah, we’ve all seen the functional criteria: metabolism, self-regulation, growth, responsiveness, reproduction. But structurally, everyone since Robert Hooke has known that living organisms are made of cells. For the past 60 years we’ve known that those cells wear a cell membrane, a flexibly fluid two-layer construction of partly-oily molecules designed to separate watery cell contents from watery outside.
The standard membrane model is on the left in these sketches. The zigzags represent long (15-20 carbons) hydrocarbon chains (the oily part); the red circles they’re bonded to stand for phosphate-containing groups that prefer a watery environment. The polka dots are salts and other molecules floating in water. The whole thing depends on “oil and water don’t mix.”

(diagram on the right adapted from the Stevenson paper)
But Titan’s liquid environment is hydrocarbons, non-polar and therefor inhospitable to dissolved salts. In a year-ago post I followed other people in proposing that cells living in Titan’s lakes might use an inverted membrane structure (the middle sketch). It’d separate oily inside from oily outside by interposing a thin layer of watery.
That might work on worlds whose temperature range matches ours. Stevenson and his team pointed out that it’s seriously unworkable on cold worlds like Titan (surface temp -290ºF). The watery parts would be frozen granite-hard and the oily parts would be stiff as high-grade candle wax. If there are living cells on Titan, they can’t use either two-layer membrane design as they move, grow and do those other life-ish things.
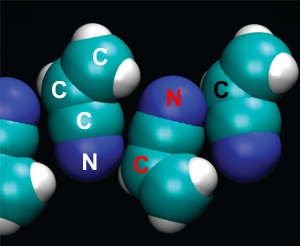
in ambipolar array
(adapted from Stevenson)
Stevenson’s team asked the next question, “What else might work?” They decided to investigate single-layer structures with no salty component at all. Such membranes could be held together by electric forces between charges that are slightly separated within the same ambipolar molecule (right-most sketch of the three above). For instance, a nitrogen atom holds onto its electrons more tightly than a carbon atom does, so a bonded C≡N pair will be slightly negative on the N side, slightly positive on the C side.
And to avoid the candle-wax problem the tails on those molecules would have to be short.
Titan’s atmosphere is 98% nitrogen and most of the rest is methane and hydrogen, so the group looked at nine ambipolar nitrogen-carbon-hydrogen molecules with short tails. For each compound they asked, “Would a membrane made of this stuff be stable on Titan? If so, would that membrane be flexible?”
Those questions are hard to answer experimentally (-290ºF is cold) so the team resorted to advanced molecular dynamics simulation programs that they just happened to have lying around because that’s what the authors do in their day-jobs.
Basically, what the programs do is arrange some virtual molecules (including solvent) in a starting configuration, then let them move around step-by-step under the influence of their mutual attractions and repulsions. Meanwhile, the programs keep track of the total energy (and a few other things) for the entire assemblage. Run the simulation until things settle down (if they do); see what virtually happens.

(also from Stevenson)
In many (not all) of the computer runs, the molecules under test did indeed form a more-or-less regular membrane floating in the “solvent.” Only some were “stable,” with calculated energies indicating they’d hold together for days or longer. Some would even be able to form hollow spheres (vesicles) at least as large as a small virus. Significantly, “flexibility” values for the stable membranes are in the same range as Earthly cell membranes.
It’s an exciting paper if you’re interested in alien life forms. Among other things, it suggests that astronomers can’t limit their surveys to planets that exhibit signs of atmospheric O2.
But Life also depends on information storage and transfer. Earth uses DNA, a huge polar molecule unsuited to Life on Titan. How might Titan’s Life handle that problem?
~~ Rich Olcott


