Long ago in a far-away career, I taught a short-course about then-current theories on the origin of life. The lab portion of the course centered on the 1952 Miller-Urey experiment the first demonstration that amino acids could be produced abiotically.
Long ago in a far-away career, I taught a short-course about then-current theories on the origin of life. The lab portion of the course centered on the 1952 Miller-Urey experiment the first demonstration that amino acids could be produced abiotically.
Imagine my surprise when I learned that Miller’s original lab apparatus is on exhibit at the Denver Museum of Nature and Science, where I volunteer now.
The diagram’s notes describe the basic experiment. Load up the system with whatever gases you think might have been in the primeval atmosphere. Start cooling water running through the condenser (double-wall tubing below the upper sphere) and gently heat the water sample you’ve put in the bottom sphere. Vapors travel up the tube into the top sphere where there’s a spark arcing between two electrodes (the black lines at 45º). Water vapor passing by sweeps any gas-spark reaction products back down to the bottom sphere.
Let the whole thing stew for a while (Miller ran his for a week, we let ours go for two), then draw off and analyze a sample of the solution in the bottom sphere. In Stanley’s day his analytical techniques found 5 amino acids. In 1971 (I think) we found (I think) 8 or 9. More recent work-ups of Miller’s sealed original samples found 25, including all 20 considered essential to life. So yeah, if you supply enough energy to a methane-ammonia-water system (Miller added hydrogen to that; we didn’t, for safety reasons) you can make the building blocks for proteins.
The experiment has been repeated probably thousands of times by different researchers in the last half-century. Some replications were duplicates of Miller’s, some started with recipes derived from other theories about what Earth’s early atmosphere looked like.
And there’s the problem. In Miller’s day we thought that Earth’s atmosphere was basically comet-tail concentrate. That’d be mostly water vapor along with a couple of volatiles like methane and ammonia. Later on we realized that much of our atmosphere is volcano belch — a hodgepodge of carbon dioxide, carbon monoxide, methane, hydrogen, sulfur gases, nitrogen, argon, helium, various acids, multiple kinds of rock dust…. Some of that is left-overs from Earth’s initial stages; some has been generated by subsequent geological processes like serpentinization, which can generate both methane and hydrogen.
If you’re running a Miller experiment you’re free to load the apparatus with whatever mixture you think other scientists might think is reasonable for an Earth on the verge of Biology. No oxygen, though — the chemistry of ancient rocks rules out significant atmospheric O2 before about 3½ billion years ago.
Now the researchers are playing variations on the theme, asking whether conditions on (or within) Titan could also generate complex compounds that given a billion years could self-organize into anything we’d recognize as life. So what did Titan’s atmosphere look like a few billion years ago?
That’s a toughie, because we don’t have on-the-ground (or out-of-the-ground) data like we have for Earth. We’ll have to make do with theory, which starts with this chart.

At any given temperature you can calculate the average energy per gas molecule (any kind of gas). Combine that with the known mass of a specific kind of molecule and you can compute its speed.
On any given world you can calculate the minimum speed (the escape velocity) that an object (rocket, rock or gas molecule) needs to have in order to overcome the world’s gravitational pull.
The chart combines both calculations for some important molecules for worlds in the Solar System that have atmospheres. For instance, Earth’s average temperature (give or take a few dozen degrees) is 300ºK=27ºC≈80ºF. From the chart, hydrogen and helium should be able to (and do) leave our atmosphere quickly. However, Earth’s gravity is sufficient to hold onto its original dowry of the heavier species. By contrast, the four massive planets would have to warm up by hundreds of degrees before they lose even the light gases.
Sure enough, Titan’s atmosphere is mostly nitrogen. The astronomers measure its methane and hydrogen content in parts per thousand but those concentrations aren’t the same going from top to bottom of Titan’s atmosphere. Therein lies an intriguing tale, but it’ll have to wait for the next post.
~~ Rich Olcott




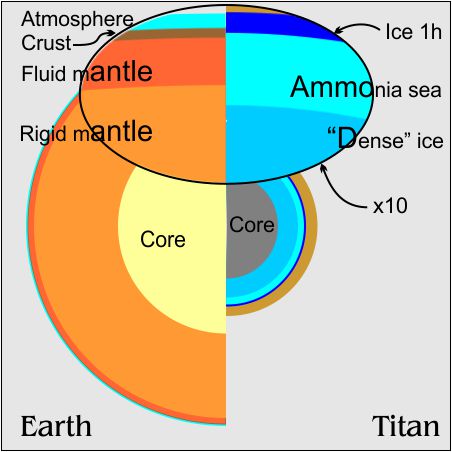

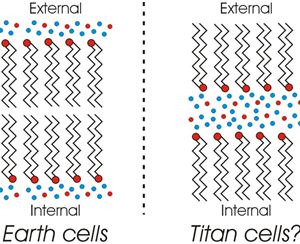
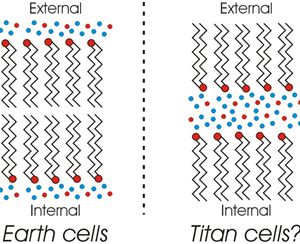
 The primary reason we think Titan is so wet is that Titan’s density is about halfway between rock and water. We know there are other light molecules on Titan — ammonia, methane, etc. We don’t know how much of each. Those compounds don’t have water’s complex phase behavior but many can dissolve in it. That’s why that hypothetical “Ammonia sea” is in the top diagram.
The primary reason we think Titan is so wet is that Titan’s density is about halfway between rock and water. We know there are other light molecules on Titan — ammonia, methane, etc. We don’t know how much of each. Those compounds don’t have water’s complex phase behavior but many can dissolve in it. That’s why that hypothetical “Ammonia sea” is in the top diagram.

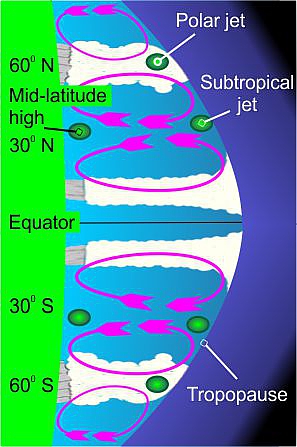 Air warmed by the equatorial Sun rises, only to sink as it heads poleward. Our packet loops between the Equator and about 30ºN (see the diagram).
Air warmed by the equatorial Sun rises, only to sink as it heads poleward. Our packet loops between the Equator and about 30ºN (see the diagram).
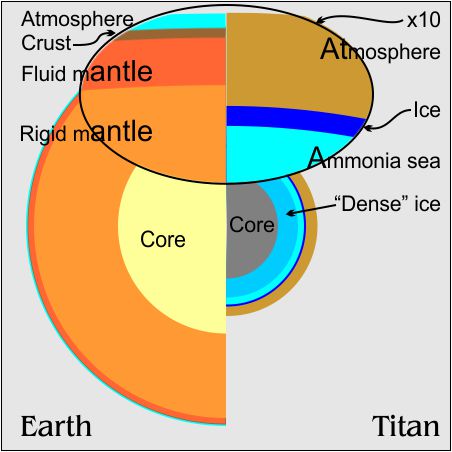 Titan’s atmosphere is heavy-duty compared with Earth’s — 6 times deeper and about 1½ times the surface pressure. When I read those numbers I thought, “Huh? But Titan’s diameter is only 40% as big as Earth’s and its surface gravity is only 10% of ours. How come it’s got such a heavy atmosphere?”
Titan’s atmosphere is heavy-duty compared with Earth’s — 6 times deeper and about 1½ times the surface pressure. When I read those numbers I thought, “Huh? But Titan’s diameter is only 40% as big as Earth’s and its surface gravity is only 10% of ours. How come it’s got such a heavy atmosphere?”

 in his Les Miz role of Inspector Javert,
in his Les Miz role of Inspector Javert, 
 And then there’s
And then there’s