Cathleen’s an experienced teacher — she knows when off-topic class discussion is a good thing, and when to get back to the lesson plan. “My challenge question remains — why isn’t Earth’s atmosphere some average of the Mars and Venus ones? Thanks to Jeremy and Newt and Lenore we have reason to expect the planets to resemble each other, but in fact their atmospheres don’t. Maria, tell us what you’ve found about how Earth compares with the others.”
“Yes, Profesora. I found numbers for many of the gasses on each planet and put them into this chart. One thing Earth is right in the middle, most things not.”
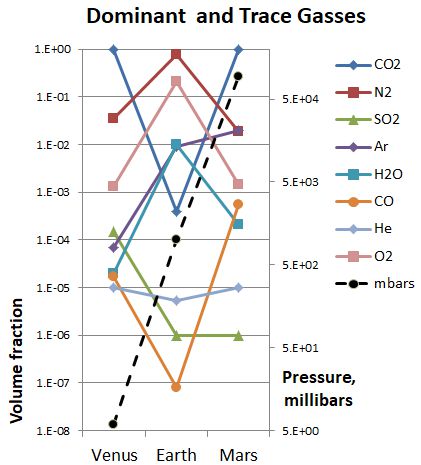
“That’s a complicated chart. Read it out to us.”
“Of course. I had to make the vertical scales logarithmic to get the big numbers and small numbers on the same chart. First is the pressure which is the black dotted line. Venus pressure at the surface is nearly 100 times ours but Mars pressure is a bit less than 1/100th of ours. Does that count as Earth being in the middle?”
“That’d be a geometric average. It could be significant, we’ll see. Go on.”
“The gas that is almost the same everywhere is helium, the grey diamonds. That surprised me, because I thought the giant planets got all of that.”
Al’s been listening in. Nothing else going on in his coffee shop, I guess. “I’ll bet most of that helium came from radioactive rocks, not from space. Alpha particles, right, Cathleen?”
Cathleen takes unexpected interruptions in stride. “Bad bet, Al. Uranium and other heavy elements do emit alphas which pick up electrons to become helium atoms. You probably remembered Cleve and Langlet, who first isolated helium from uranium ore. However, the major source of atmospheric alphas is the solar wind. Solar wind interception and atmosphere mass are both proportional to planetary surface area so a constant concentration like this is reasonable. Continue, Maria.”
“The major gasses follow a pattern — about the same fractions on Venus and Mars but much higher or lower than on Earth. Look at carbon dioxide, nitrogen, even oxygen.”
Astronomer-in-training Jim has been doing some mental arithmetic. “Our atmosphere is 100 times denser than on Mars, and Venus is another factor of 100 beyond that. That’s a factor of 104 between them — for every molecule of CO2 on Mars there’s 10,000 on Venus. Oh, but Venus has four times Mars’ surface area so make that 40,000.”
“Good points, both of you. Jim’s approximation leads into something we can learn from Maria’s trace gas numbers. Why do you suppose the concentration of SO2 is about the same for Earth and Mars but 100 times higher on Venus, but the reverse is true for argon? Where do they each come from?”
Jeremy finally has something he can contribute. “Volcanoes! They told us in Geology class that most of our SO2 comes from volcanoes. Before the Industrial Revolution, I mean, when we started burning high-sulfur coal and fuel oils and made things worse. Venus has to be the same. Except for the industry, of course.”

“Probably correct, Jeremy. From radar mapping of Venus we know that it has over 150 large volcanoes. We don’t know how many of them are active, but the Venus Express spacecraft sent back evidence of active vulcanism. In fact, Venus’ SO2 score would probably be even higher if much of its production didn’t oxidize to SO3. That combines with water to form the clouds of sulfuric acid that hide the planet’s surface and reflect sunlight so brightly.”
Maria’s hand is up again. “I don’t understand argon’s purple diamonds, profesora. I know it’s one of the inert gasses so it doesn’t have much chemistry and can’t react into a mineral like CO2 and SO2 can. Shouldn’t argon be about the same on all three planets, like helium?”
“Mm-hm, argon does have a simple chemistry, but its radiochemistry isn’t so simple. Nearly 100% of natural argon is the argon-40 isotope created by radioactive decay of potassium-40. Potassium is tied up in the rocks, so the atmospheric load of argon-40 depends on rocky surface erosion. Not much erosion, not much argon.”
Al’s on tenterhooks. “All this is nice, but you still haven’t said why Earth’s atmosphere is so different.”
~~ Rich Olcott










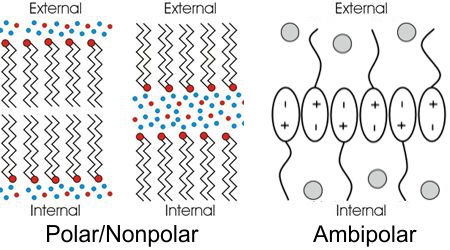

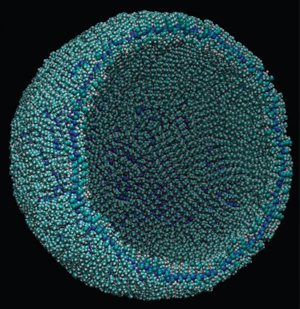


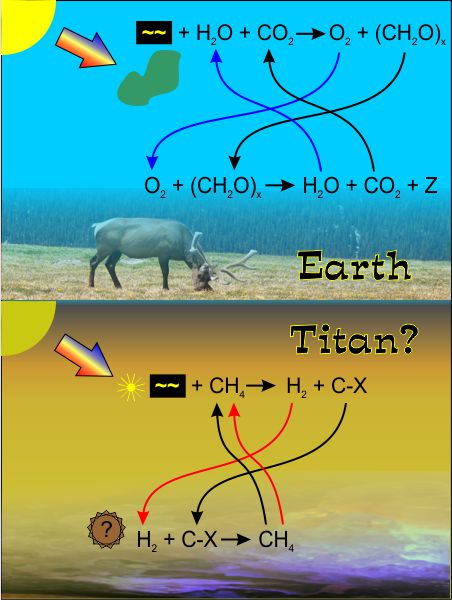

 Long ago in a far-away career, I taught a short-course about then-current theories on the origin of life. The lab portion of the course centered on the 1952
Long ago in a far-away career, I taught a short-course about then-current theories on the origin of life. The lab portion of the course centered on the 1952 

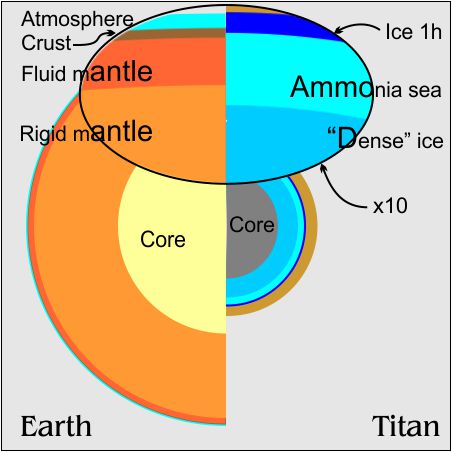

 The primary reason we think Titan is so wet is that Titan’s density is about halfway between rock and water. We know there are other light molecules on Titan — ammonia, methane, etc. We don’t know how much of each. Those compounds don’t have water’s complex phase behavior but many can dissolve in it. That’s why that hypothetical “Ammonia sea” is in the top diagram.
The primary reason we think Titan is so wet is that Titan’s density is about halfway between rock and water. We know there are other light molecules on Titan — ammonia, methane, etc. We don’t know how much of each. Those compounds don’t have water’s complex phase behavior but many can dissolve in it. That’s why that hypothetical “Ammonia sea” is in the top diagram.