There’s a commotion in front of Al’s coffee shop. Perennial antiestablishmentarian Change-me Charlie’s set up his argument table there and this time the ‘establishment’ he’s taking on is Astrophysics. Charlie’s an accomplished chain-yanker and he’s working it hard. “There’s no evidence for dark matter, they’ve never found any of the stuff and there’s tons of no-dark-matter theories to explain the evidence.”

Big Cap’n Mike’s shouts from the back of the crowd. “What they’ve been looking for and haven’t found is particles. By my theory dark matter’s an aspect of gravity which ain’t particles so there’s no particles for them to find.”
Astronomer-in-training Jim spouts off right in Charlie’s face. “Dude, you can’t have it both ways. Either there’s no evidence to theorize about, or there’s evidence.”
Physicist-in-training Newt Barnes takes the oppo chair. “So what exactly are we talking about here?”
“That’s the thing, guy, no-one knows. It’s like that song, ‘Last night I saw upon the stair / A little man who wasn’t there. / He wasn’t there again today. / Oh how I wish he’d go away.‘ It’s just buzzwords about a bogosity. Nothin’ there.”
I gotta have my joke. “Oh, it’s past nothing, it’s a negative.”

“Come again?”
“The Universe is loaded with large rotating but stable structures — solar systems, stellar binaries, globular star clusters, galaxies, galaxy clusters, whatever. Newton’s Law of Gravity accounts nicely for the stability of the smallest ones. Their angular momentum would send them flying apart if it weren’t for the gravitational attraction between each component and the mass of the rest. Things as big as galaxies and galaxy clusters are another matter. You can calculate from its spin rate how much mass a galaxy must have in order to keep an outlying star from flying away. Subtract that from the observed mass of stars and gas. You get a negative number. Something like five times more negative than the mass you can account for.”
“Negative mass?”
“Uh-uh, missing positive mass to combine with the observed mass to account for the gravitational attraction holding the structure together. Zwicky and Rubin gave us the initial object-tracking evidence but many other astronomers have added to that particular stack since then. According to the equations, the unobserved mass seems to form a spherical shell surrounding a galaxy.”
“How about black holes and rogue planets?”
Newt’s thing is cosmology so he catches that one. “No dice. The current relative amounts of hydrogen, helium and photons say that the total amount of normal matter (including black holes) in the Universe is nowhere near enough to make up the difference.”
“So maybe Newton’s Law of Gravity doesn’t work when you get to big distances.”
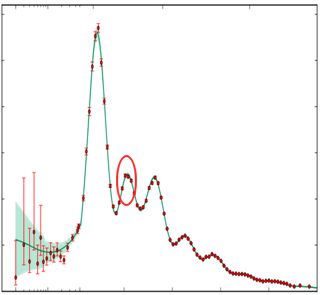
“Biggest distance we’ve got is the edge of the observable Universe. Jim, show him that chart of the angular power distribution in the Planck satellite data for the Cosmological Microwave Background.” <Jim pulls out his smart-phone, pulls up an image.> “See the circled peak? If there were no dark matter that peak would be a valley.”
Charlie’s beginning to wilt a little. “Ahh, that’s all theory.”

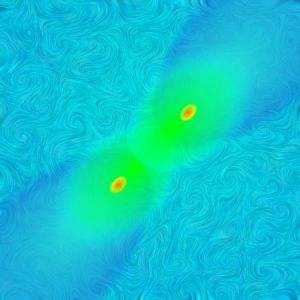
<Jim pulls up another picture.> “Nope, we’ve got several kinds of direct evidence now. The most famous one is this image of the Bullet Cluster, actually two clusters caught in the act of colliding head-on. High-energy particle-particle collisions emit X-rays that NASA’s Chandra satellite picked up. That’s marked in pink. But on either side of the pink you have these blue-marked regions where images of further-away galaxies are stretched and twisted. We’ve known for a century how mass bends light so we can figure from the distortions how much lensing mass there is and where it is. This picture does three things — it confirms the existence of invisible mass by demonstrating its effect, and it shows that invisible mass and visible mass are separate phenomena. I’ve got no pictures but I just read a paper about two galaxies that don’t seem to be associated with dark matter at all. They rotate just as Newton would’ve expected from their visible mass alone. No surprise, they’re also a lot less dense without that five-fold greater mass squeezing them in.”
“You said three.”
“Gotcha hooked, huh?
~~ Rich Olcott



 “That was a most excellent meat loaf, Sis. Flavor balance was perfect.”
“That was a most excellent meat loaf, Sis. Flavor balance was perfect.”

 “Not much. We can’t see it, and they say there is much more of it than the matter we can see. If we can’t see it, how did she find it? That’s a thing I don’t understand, what I came to your office to ask.”
“Not much. We can’t see it, and they say there is much more of it than the matter we can see. If we can’t see it, how did she find it? That’s a thing I don’t understand, what I came to your office to ask.”



 The thing about Al’s coffee shop is that there’s generally a good discussion going on, usually about current doings in physics or astronomy. This time it’s in the physicist’s corner but they’re not writing equations on the whiteboard Al put up over there to save on paper napkins. I step over there and grab an empty chair.
The thing about Al’s coffee shop is that there’s generally a good discussion going on, usually about current doings in physics or astronomy. This time it’s in the physicist’s corner but they’re not writing equations on the whiteboard Al put up over there to save on paper napkins. I step over there and grab an empty chair.

 “Hey, that’s the
“Hey, that’s the